| Whoa! Hold on! How much thinner do you plan to add? What thinner do you plan to add? The data sheet says you can add up to 10% by volume.Even so, it is not uncommon to see painters (or painters’ helpers) estimating thinner addition. It takes an experienced applicator who can save precious time by estimating thinner additions. After all, the reducer is going to evaporate from the applied coating film anyway. Should it matter that the solvent is supplied by “Universal Solvent,” and the coating is supplied by “Not-So-Universal Resin?” Of course it matters, just as it matters when adding 15% thinner when 5% will produce the desired viscosity. When was the last time you actually saw a bridge painter measure viscosity? And who actually mixes the paint? The person with twenty years of experience or the person who has had one year of training twenty times?
As seen in JPCL, March 2014 Solvents in CoatingsSolvents are among the liquid components of coatings commonly referred to as the volatile vehicle. Upon application, and for some time afterward, the solvents evaporate, leaving the vehicle solids behind, which form the dry and cured coating film. The volatile vehicle(s) should be understood to be transitory components used in liquid coatings because of two principal properties: solvency and release rate. The first, solvency, relates to the ability of any given solvent to dissolve a resin and produce a homogeneous solution. Solvency is also an important property because it controls the coating’s viscosity for application purposes. The second property, release rate, is important for the film-forming properties of the coating. The applied coating film may require both rapid and slow solvent release. Rapid release is primarily due to evaporation, the first stage of solvent release. Slow release is primarily controlled by solvent diffusion from the applied coating film. Evaporation allows the film to build and achieve the targeted wet film thickness (WFT) without running or sagging. However, slower release (diffusion) allows the coating to wet-out on the substrate (promoting adhesion) and also allows the atomized coating to flow-out into a uniform film. Therefore, solvents are formulated into coatings to provide for a liquid application that “dries” at a rate that determines film build and surface wetting. We are all aware that additional solvents (typically called thinners) are sometimes added during the mixing of coatings prior to application. Thinning is typically done to improve the application properties of the coating by reducing its viscosity. However, thinning is not always necessary, and in some cases, it is frowned upon, as some instructions specify, “Do not thin coatings without approval of the engineer.” Coating manufacturers may clearly state that thinning is not normally necessary for application of their product, but if you must use thinner, instructions often read: “Use no more than X% by volume of thinner Y.” Coating manufacturers not only advise about how much thinner to add, but also which solvent product(s) may be used. Several products may be listed for the same coating. Determining solvent selection will depend on the resin type, the ambient temperature, and the volatile organic compound (VOC) content (which is limited by regional regulatory standards). The most critical of these influences is resin type. The thinners to be added must fall within a solvency window for dissolution. All thinners are not suitable for all resins, and vice versa. Ambient temperature is also important to solvent selection. For example, a product data sheet might require that in temperatures less than 80 F, use thinner “L”, and at temperatures higher than 80 F, or for windy conditions, use thinner “H.” The key property is related to the rate of solvent evaporation. Such requirements demonstrate that there are different solvents available for thinning a given coating resin system. The difference is related to temperature and its influence on the evaporation rate and diffusivity of “fast” and “slow” solvents. Use of solvent “L” in aliphatic polyurethanes on hot days or surfaces can result in a film with hundreds of small pinholes. It is possible that the solvents used may be the same in both cases, their ratios may be quite different. Therefore, one can easily conclude that there must be different solvent blends or “packages” available. One coating manufacturer’s solvent blend may be slightly different than the solvent blend from a different manufacturer, hence coating manufacturers’ recommendations to use only their own thinners. Hopefully, the discussion above sheds some light not only on why we see limits on the types and amounts of thinner recommended by coating manufacturers in product data sheets, but also on why thinning may be further limited or even prohibited in project specifications. We assume that the solvents added during the manufacture of coatings are correct in type and amount and, as a general rule, that coatings thinned and applied per the manufacturer’s instructions will form the desired film properties. Yet somehow, solvent use still can cause problems, and solvent entrapment can be a significant one. Types of SolventsThere are numerous types of polymers (resins) used in the manufacture of coatings, and nearly all polymers must be synthesized in organic solvents. The solubility of the polymer in the appropriate solvent (and/or solvent blend) is necessary for the coating to be properly manufactured and applied. There are three major classifications of solvents used in coatings: petroleum hydrocarbon solvents (aliphatic solvents such as hexane and mineral spirits and aromatics solvents such as xylene and toluene), terpene solvents (derived from pine trees by solvent extraction and distillation), and oxygenated solvents (ketones, esters, alcohols, and glycol ethers). The solvent strength as well as the polymer solubility are key parameters. Waterborne coatings differ from solvent-borne coatings because polymers in waterborne coatings are not actually dissolved in water. For example, waterborne latex coatings are actually dispersions of high molecular weight polymers throughout the water. Solvent Release—Evaporation and DiffusivitySolvent release from a coating should be viewed as a two-stage process. The first stage is related to evaporation rate, which is a function of volatility, temperature, and vapor pressure (of the solvent) at the surface. The second stage is the diffusivity rate. Solvent loss can be hindered by increasing the viscosity of the film and the degree of curing, such as in crosslinking, which is an ongoing process. Think of the film matrix as a room with many people dressed in black (curing resin) and some people dressed in white (solvent). In order for people dressed in white to leave the room, they must move between people dressed in black, from open space to open space (or in the case of solvents in coatings, from free volume to free volume). Add Velcro strips to the outside of the black clothes. When the people dressed in black congregate into a tight group, the open spaces become smaller in size. Because of “Velcro bonding,” they begin to crosslink, making it more and more difficult for people dressed in white to reach the door. They have to squeeze their way through the tight group to get out. Some may take a very long time to get out, and some may never get out. Waterborne coatings are somewhat of a different model. Instead of being dissolved, the resin is dispersed in water, but it still will not form a film until much of the water has evaporated. In addition to water, co-solvents are added to waterborne coatings to perform very specific functions. The evaporation rates of water and co-solvent (coalescing aid or agent) are equally critical. The co-solvents used are typically slow-evaporating solvents of glycol esters or ethers. It is important that these constituents be available, as the bulk of the water evaporates to aid in the coalescence of the dispersed resin. High humidity and low temperatures retard the evaporation of water, but not necessarily the co-solvent. With insufficient coalescence, many desired film properties are lost. Rapid loss of water also presents a problem because the amount of co-solvent relative to the amount of water becomes increasingly elevated, and the dispersion may collapse. Solvent EntrapmentSolvents may become trapped in a coating film. The root cause may be low temperature, thus, a lower evaporation rate, or an overly thick coating film requiring high diffusivity. Simply put, much of the volatile vehicle fails to evaporate or diffuse from the film. The specific causes and consequences of solvent entrapment are discussed below. Consequences of Solvent Entrapment• Bubbling: Solvents that are trapped in coating films can form bubbles in the coating as it cures. When seen in cross-section, the bubbles actually are voids in the coating film (Fig. 1). In some instances, bubbles migrate to the surface and leave a crater, if the coating film has gelled too much to flow in behind and fill it. Overly thick moisture-curing or oxidation-curing coatings are susceptible to forming such bubbles because the surface of the coating film begins to “skin” over, making evaporation and diffusion increasingly difficult. Coating applications at high temperatures will cause bubble formation, as will excessive amounts of slow-evaporating thinner in cool weather. Bubbling can be minimized by reducing the coating to the proper viscosity and applying it at the recommended temperatures.
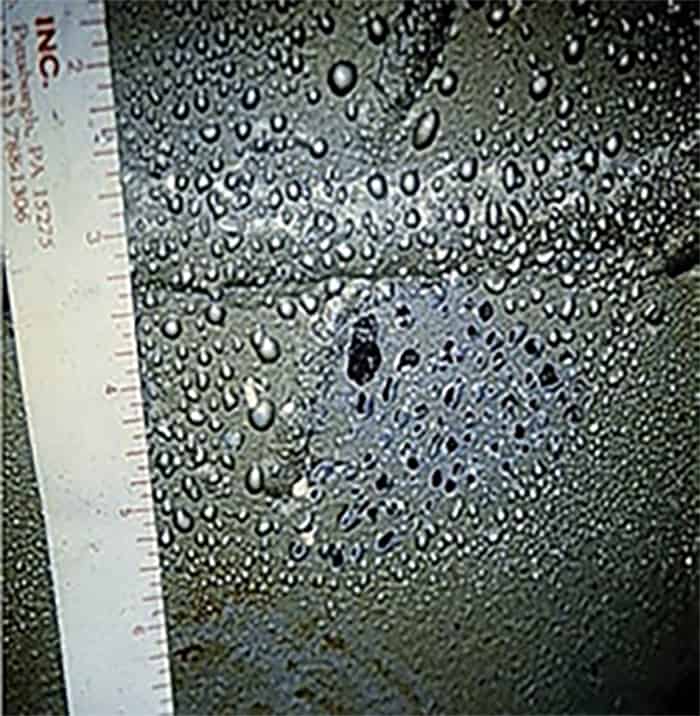
• Pinholes: Pinholes may result from solvent bubbles that migrate through a partially gelled film that does not have sufficient flow to close in behind the trail that is made in the film (Fig. 2). As described for craters on the surface, the void left open in this case may extend through the coating system to the substrate. This void leaves an avenue for moisture and contaminants to cause corrosion. Even with the application of a second coat the pinholes may telegraph through to the next layer.
• Cracking: Cracking is typically recognized as a consequence of internal coating stress. There is more than one mechanism involved. The solvent-related mechanism is typically related to the surface of an overly thick coating that is gelling or skinning over while slow diffusion results in a softer matrix below the surface. The surface shrinks and splits as if a hard coating was applied over a softer, more flexible film. The related cracking can also result when a second coat is applied over a wet coat (wet on wet application). Diffusion path length is increased and free volume decreases from the top down. Coatings that cure by oxidation or moisture cure from the top down and are quite susceptible to solvent entrapment. Alligator cracking typically occurs at the surface and extends into the softer coating layer beneath (Fig. 3).
• Orange Peel: Orange peel is the result of poor flow-out of the coating film. Insufficient solvent or incorrect solvent use can inhibit leveling of the coating surface, leaving a bumpy, dimpled texture that looks like an orange. Orange peel is often associated with thick films whose surfaces fail to flow out uniformly. This condition is not n ecessarily a cause for coating removal or repair because the film may still function properly. It is, however, an indication that thinning and/or application adjustments are needed. • Flocculation: Flocculation occurs when resin polymers within the liquid coating agglomerate into large particles rather than remain in suspension or solution. Flocculation due to a solvent problem is most always associated with the use of an incorrect solvent. The polymers may not be soluble in the added solvent or the combination of the volatile vehicle results in a lower solvency. Polymer(s) drop out of solution generally resulting in growing clumps of a gelled or gelatinous matrix. • Running and Sagging (Curtains): The development of runs and sags on vertical surfaces is related to low viscosity of the coating film. Runs are thin streaks of coating that flow down the surface and subsequently solidify. While this is primarily an aesthetic issue, the thick runs may also crack. Sags (or curtains) occur when an area of coating flows to form a thicker film at the base than the coating above. Although not solely caused by excessive thinning, sags are consequences of overly thinned coatings. The film build normally expected cannot be achieved, and running or sagging result. Incorrect mix ratio, overly warm coatings (which have a lowered viscosity), or coatings applied too thick can also lead to sags. • Blistering: Blisters in coating films may be the result of several solvent-related issues. Baked coating films may form blisters or exhibit solvent “popping” because the free volume at the coating surface is reduced at an accelerated rate. Solvent release becomes much more dependent on diffusion than evaporation. Solvents accumulate within the available (decreasing) free volume of the curing film. The higher temperatures of baking make solvents more active (resulting in increased internal pressure), and they will form blisters if unable to diffuse. Osmotic blistering is typically associated with soluble salts trapped beneath a coating. The imbalance of ionic strength causes water to move through a semi-permeable membrane, effectively increasing the volume of trapped water resulting in a blister. The same phenomena results when solvents are trapped in a coating film. Just as soluble salts are hydrophilic, solvents that are hydrophilic may be included in coating formulations. The solvents (alcohols and glycol ethers and their acetates) become trapped, occupy volume, and draw water into the film (Fig. 4). The most severe occurrences are related to coatings in immersion service.
• Solvent Sensitivity: Two solvent-induced problems affecting aesthetics are bleeding and blooming. Bleeding is used to describe a discoloration of an upper layer coating layer as a result of coloring matter from a lower layer (which can also be the substrate) diffusing to the surface of the topcoat. It is most pronounced on lighter color topcoats but is not exclusive to light colors. The diffusion may be the result of solvents in the topcoat. Ho wever, in the case of waterborne coatings, moisture from the environment may be a contributing factor. Discoloration is the major problem. Blooming is related to bleeding but is associated with a reduction in gloss. Components in an applied film are leached into an upper layer of the topcoat by solvents. Upon reaching the surface, the solvents leave the film, leaving the leached products (exudate) on the surface. Solvent-sensitive pigments can result in a splotchy appearance on the surface with a corresponding change in gloss and modest or negligible color change. There is a bloom that is characteristic of waterborne latex formulations where the exudate is attributed to surfactants and/or dispersants in the film. Sampling and Testing for Entrapped SolventsTesting for residual solvents in a coating may be as simple as sniffing the coating chip or the surface where some of the coating has been removed. This method is quick and easy, but it also is subjective and depends heavily on the sensitivity of the “sniffer.” Confirmatory, more objective testing is available through sample collection and analysis of coating chips in the laboratory. Sampling for the presence of solvents in coating films or blister liquids must be performed with care to avoid contamination, loss of solvent, or solvents extracting material from the (plastic) sample container. Coating chips can be removed from the surfaces using a knife, chisel, scraper, or similar tool. The chips may be sniffed for solvent. However, the solvents can evaporate quickly, so the chips should be immediately placed into a septum vial (to avoid solvent loss) and the lid secured. In the absence of a glass vial or tight seal, the chips may be tightly wrapped in clean aluminum foil. Liquids can be removed from intact blisters to determine if there are solvents in the solution. Frequently, salts are the first materials tested for. However, the ability of water-miscible solvents to create osmotic blisters should never be overlooked. The blister surfaces should be cleaned of dirt and debris to avoid contamination. Once cleaned, the blister may be punctured using a knife, large pin, or finishing nail, each of which should also be clean. A syringe is placed through the hole after the plunger is depressed, and the plunger is then pulled back to suck blister liquid into the barrel. Most syringes used in the field are plastic, so the fluid collected should be transferred to a glass septum vial as soon as possible (Fig. 5). It should be noted that multiple blisters may need to be sampled to obtain sufficient volume of blister liquid for testing.
The coating chips or blister liquid samples can be accomplished by gas chromatography (GC) or GC-mass spectroscopy (GC-MS). The sample is heated, causing the solvents present to evaporate or diffuse from the sample. An inert carrier gas flowing through the system carries the compounds, leaving the sample into the column of the GC. The column, having various affinities to solvents, separates the solvent mixture (e.g. by boiling point) so that the individual solvent compounds are detected at different time intervals. An example of the chromatogram produced is provided in Fig. 6. Note that the table in Fig. 6 identifies some of the compounds found in the sample.
ConclusionThe next time you hear, “no one measures thinner addition” or “MEK is a universal solvent,” you’ll know the possible consequences of adding too much thinner or the incorrect type of thinner to a coating. It takes a few more minutes to do it right, and doing it right can save thousands of dollars in rework. There are many circumstances that can create problems during industrial painting. Doesn’t it make sense to minimize those that are self-inflicted?
|








Congratulation, as senior consultant and ancient director of “chemistry formulation” , i.e. paints, inks, adhesives, cosmetics, @ ITECH-Lyon, in University of Lyon in France, I do agree with this wonderful paper.
We appreciate your endorsement Yves. Thanks for reading! Feel free to share this article with your colleagues and peers.
-Jason, KTA Marketing Department
A great article. I believe this is a crucial step toward the success of any given project and should be taken as such. There are, as you mentioned, a host of problems that can occur indirectly because of poor mixing practices. I would like to add, however, the effects that temperature has on the material itself, and its relation to viscosity. Ambient temperatures, humidity, and surface temperatures are key factors to consider when selecting an appropriate solvent, and how much we are to add. Even during “normal” applications, if the material temperature is below what is normally recommended by the manufactures the tendencies of the painter, or painter’s helper, is to add more solvent in order to achieve the desired flow necessary for an efficient application. This can be easily avoided as well by maintaining proper storage conditions, as recommended by manufacturers. Also, heating the material to increase the viscosity may reduce the need for solvent completely in some applications. This might also be something to consider if you are trying to manage the VOC’s of an applied material. That being said storage temperatures and application temperatures need to be properly monitored as well and should be done in accordance with the manufacturer’s recommendations.
THE BEST I HAVE READ SO FAR IS YOUR ARTICLE AND I NEED TO READ MORE OF THIS.
THANKS FOR ALL YOUR EXPLANATIONS.