At its very basic level, saponification is the term given to the process in which vegetable oils and animal fats are converted into soap. We recognize soaps as wax-like products, modified through the addition of fragrances and skin-care additives (emollients) to become consumer cleaning and bathing products. The essential step in the soap-making process is the incorporation of an alkaline substance, like lye (sodium hydroxide, NaOH). The lye provides a source of hydroxyl ions (OH-) that digest and break down the structure of the oil or fat and produce various triglycerides. An example is shown in Figure 1.
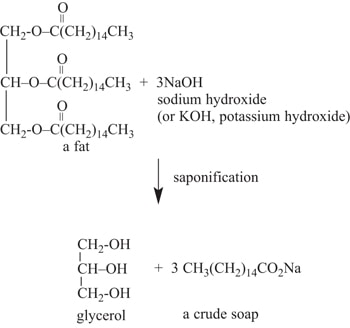 This article explores the many facets of saponification, including the chemical and environmental conditions that must be present for saponification to occur, and how it contributes to paint adhesion failures. A real life example of a saponification failure is discussed in-depth to aid in understanding the process. From Paint: The Destructive Nature of SaponificationSaponification can be destructive to paint films. Oil-based paints that utilize alkyd esters, epoxy esters, or linseed oil derivatives in their composition can saponify when exposed to an alkaline environment and moisture. Some polyvinyl acetate (PVA) water-borne latexes can also be broken down by saponification. The saponification reaction forms water-soluble soaps at the interface between the coating and metallic or masonry substrate. These soaps become soft, sticky, and dissolve when exposed to moisture. The moisture can be present in the original wet coating; as atmospheric humidity that passes through the cured coating film and reaches the alkaline substrate; or, in the case of cementitious materials, as free moisture from the mix, ground water, or leaks (water intrusion). Some alkyd formulations will not undergo saponification. These formulations incorporate a modified alkyd. For example, modifications that employ diisocyanate as a substitute for dibasic acids result in products commonly referred to as uralkyds. Ester groups are replaced with a urethane linkage and superior alkali resistance compared to conventional alkyds. Modified alkyds can even be blended with Portland cement or zinc/zinc pigments. Other coating binders that resist saponification include acrylic and solvent-borne epoxy formulations. Saponification of Painted Masonry SurfacesMasonry surfaces with an alkaline condition include new concrete, tilt-up slabs, stucco, plaster, and mortar. These masonry surfaces are characteristically alkaline, with a pH range of 8 to 14. For example, freshly poured concrete and plastered surfaces initially have a very high pH that can range from 12 to 14. These surfaces are sometime referred to as “hot” by coating applicators. As the masonry surfaces cure, the pH range will drop to a range of 10 to 12. However, the surfaces will still be prone to saponification if oil-based or conventional alkyd coatings are used. Saponification of Painted Metallic SurfacesMetals that have an alkaline surface condition include zinc (galvanizing), copper, magnesium, tin, and lead. Painting over ferrous metals such as mild, carbon, and stainless steels or iron will not cause saponification. Iron in solution does not produce an alkaline environment. A Pourbaix diagram is used to predict the tendency of a metal surface to develop alkaline condition in an aqueous environment. Figure 2 is a Pourbaix diagram that shows the zinc species (compounds) that will be present in the solution. Zinc, being amphoteric, is soluble in acid and base (alkaline) solutions, but the species present will be a function of equilibrium between the zinc species. The passive zone (pH 8.5–10.5) is where a surface layer of zinc hydroxide (ZnOH2) forms and zinc metal corrosion does not occur. It is important to note that this exists within the stable range for water (the area within the dotted lines) while exhibiting a pH level of 8.5 to 10.5. Beyond this region, corrosion occurs at higher pH values and will produce saponification. Recall from Figure 1that it is the hydroxide ions (OH-) that react with the oil, alkyd, or fat to form glycerides and soaps. Excess OH- ions are what makes an aqueous solution basic just as excess H+ ions make a solution acidic. A similar Pourbaix diagram can be drawn for copper, lead, magnesium, and tin metal surfaces in an aqueous (water) environment.
A Real World Example of SaponificationThe interior surfaces of galvanized steel roof decking applied in a large warehouse were painted by the direct application of two different dry fall alkyd coating systems. Coating A was an alkyd coating modified with approximately 15% Portland cement. Coating B was an epoxy ester (modified alkyd) dry fall coating. Both coating systems were essentially 50% solid, single-component formulations with a solvent blend of hydrocarbons. The solvent phase was designed to evaporate so rapidly that any overspray would become a dry powder as it fell through a vertical distance of eight feet. Both Coatings A and B were recommended by the coating manufacturer as suitable for application to galvanized metal substrates. The work location was in Fort Walton Beach, FL.
The dry fall coatings were spray applied at a DFT range of 3–8 mils and reached a full cure in seven days at 77 F. Following the spray application, there were no apparent problems. However, within several months of application, delamination was observed on the interior galvanized deck ceilings that were coated with the epoxy ester dry fall coating (Coating B) and within the immediate vicinity of a series of roll-up doors (Fig. 3). The surfaces painted with Coating A showed no signs of distress. Investigating the Failure
The field investigation consisted of visual observations, dry film thickness measurements, and adhesion tests. Samples of the coating also were collected for laboratory analysis. Dry film thickness measurements were obtained in general accordance with ASTM D7091, Standard Practice for Nondestructive Measurement of Dry Film Thickness of Nonmagnetic Coatings Applied to Ferrous Metals and Nonmagnetic, Nonconductive Coatings Applied to NonFerrous Metals. Dry film thickness measurements of the exposed galvanized steel were taken and then deducted from the total system thickness that was measured. Adhesion tests were conducted in accordance with ASTM D3359, Standard Test Methods for Measuring Adhesion by Tape Test. The test involved making an X-scribe in the paint film. An adhesive tape was applied to the scribe and then lifted off of the surface. Adhesion was rated on a scale of 0A to 5A according to the amount of coating removed by the tape, with 5A being best (no coating removed). Field Investigation Results
Essentially, the field investigation revealed the following.
Laboratory Investigation Results
Infrared (IR) spectroscopy was used to analyze the failed coating and the waxy substance found on the back of the failed Coating B removed from the galvanized surfaces on the interior decking (Fig. 5). A control sample supplied by the coating manufacturer was also examined by IR spectroscopy. The analyses determined that the failed coating and control sample of the coating were consistent in formulation with a calcium carbonate-filled epoxy ester coating. Analysis of the waxy material at the interface of the delaminated coating was found to be consistent with the carboxylic acid salts produced by alkaline hydrolysis of an ester.
RecommendationsSurfaces containing the failed epoxy ester coating were to be washed with pressurized water with enough pressure (5,000–10,000 psi) to remove all of the deteriorated coatings. It was anticipated that this process would remove all of the saponification products. The pressurized water washing was to be supplemented by brushing and scrubbing incorporated with an aqueous cleaning agent. Following cleaning, the surfaces were to be inspected to verify that no artifacts of saponification remained. Once the surfaces were prepared, a primer specific for galvanizing was to be applied and then overcoated with one or two coats of a 100% acrylic dry fall coating. ConclusionsA unique characteristic of the failed coating areas, and the actual cause of the coating failure, was the presence of a deposit on the epoxy ester coating in the failed areas. Infrared spectroscopy revealed that the deposit was consistent with carboxylic acid salts—a reaction product from the alkaline hydrolysis of epoxy esters (saponification). Epoxy esters, oil and alkyd resins can saponify when applied to galvanized surfaces that experience humid conditions. The source of the alkaline surface is the galvanizing itself. The alkaline nature of the zinc surface can be predicted by the Pourbaix diagram for zinc metal in an aqueous environment (Fig. 2). This reaction forms a soap-like material at the coating/substrate interface and eventually causes the coating to delaminate from the substrate. Epoxy esters are oil-based coatings with epoxy resins. Since humidity is essential for the saponification reaction to occur, it is not unexpected that the most prevalent failures were in areas adjacent to the roll-up doors. Further inside the buildings, where HVAC limited humidity, it was demonstrated that the epoxy ester coating could be used successfully over the galvanized surfaces. To Grave—A Bizarre Example of SaponificationOddly, the same process of converting animal fat to soap has also been documented with human corpses. The Mütter Museum in Philadelphia, PA, has on display an exhumed human corpse, originally buried in 1792, that was converted to adipocere. Adipocere, also known as corpse wax or the fat of graveyards, is a product of decomposition that turns body fat into a soap-like substance. Corpse wax is the result of saponification when body fat is exposed to anaerobic bacteria in a warm, damp, alkaline environment. Saponification will stop the decay process in its tracks by encasing the body in this waxy material, turning it into a “soap mummy.” The wax is ester of alcohol and fatty acids. They differ from fats since they don’t have triglyceride ester of three fatty acids which can be attributed to the anaerobic bacteria. While somewhat bizarre, the event again demonstrates how saponification will convert any oil or fat into soap.
|







Thanks for a thorough article; it was just what I needed for an explanation during a recent specification debate.
No problem, Josh. Glad KTA could help!