KTA’s Certified Coating Inspector Forum Issue No. 8 – December 2022
KTA’s Certified Coating Inspector Forum is designed to provide professional development/continuing education on standards, inspection practices, new instruments, and other topics to help keep certified AMPP and FROSIO coating inspectors current. It represents the views of the author and KTA-Tator, Inc. It may or may not represent the views of AMPP: The Association for Materials Protection & Performance, even though SSPC, NACE, and AMPP standards are frequently referenced in the content.
Introduction
All too often, coating inspector training courses focus on the more common inspection checkpoints such as measuring ambient conditions, surface profile, and dry film thickness since these are frequently invoked in specifications, independent of the structure type and service environment. However, as trained and certified coating inspectors we are expected to be knowledgeable and proficient in all types of inspections, whether they are common or not. Surface soluble salt contamination detection and analysis is one of those “less frequently” invoked requirements but following proper procedures for testing and reporting of results can make or break an industrial/marine coating project. This article is written to convey why this type of testing is important and explores various methods of soluble salt extraction and analysis performed in the shop or field, and the frequency of testing.
Background (Why this is Important)

Chemical contaminants on a surface can include chloride, ferrous ions, sulfates, and nitrates, among others. These chemicals or “salts” are deposited onto surfaces while the structure is in service, or during the transportation of new steel to or from the fabrication shop. They are soluble in water, so they can typically be removed from surfaces by pressure washing or water jetting using clean water (hot water works better than cold) or water with the addition of a proprietary salt removal-enhancement solution. The effectiveness of the washing step is dependent on the condition of the surface. That is, contamination is relatively easy to remove from smooth surfaces, but may be more challenging if the surfaces are pitted or crevices are present, as contamination will tend to concentrate in these areas. If the salts are not detected or are not adequately dissolved and rinsed from the surfaces, they can become trapped beneath a newly installed coating system. If there is sufficient water in the service environment, (e.g., immersion) the water-soluble contaminant trapped beneath the coating system will draw the water through the coating film by a process known as “osmosis.” This drawing force can be quite powerful and will continue until the concentration of salt in water is the same on both sides of the coating film (the concentration reaches equilibrium). This process creates a build-up of water and pressure beneath the coating film, oftentimes enough to cause blistering of the coating (known as osmotic blistering), under film corrosion, and premature coating failure.
Additionally, if soluble salts on the surface are not sufficiently removed prior to abrasive blast cleaning, recycled abrasive media can become contaminated. Note that SSPC-AB 2[1] requires that recycled abrasive be routinely tested for water-soluble contaminants (maximum concentration of 1,000 µS/cm).
It is for these reasons that some specifications require inspection of surfaces for chemical contaminants before and/or after surface preparation operations are complete, but before primer application. Because this type of contamination cannot be detected visually, the surface must be sampled, and the “extract” tested for the contaminant(s) of concern.
Note that although salt contamination on freshly blast-cleaned steel cannot be seen visually, if the steel remains uncoated overnight, the effect of the salts is often visible the next day as discrete spots of rust across the surface, typically associated with pits. If a salt-contaminated surface is painted very quickly after blasting, the rusting will not yet be visible so it will be assumed that everything is fine, but potentially detrimental salts are now trapped beneath the coating film. Testing can help prevent this from happening.
Industry Standards
Existing industry standards are primarily focused on surface extraction and analysis procedures, as well as frequency and locations of testing. Common standards include:
- SSPC Guide 15, Field Methods for Retrieval and Analysis of Soluble Salts on Steel and Other Nonporous Surfaces
- SSPC Guide 24, Soluble Salt Testing Frequency and Locations on New Steel Surfaces
- NACE SP-0716-2016, Soluble Salt Testing Frequency and Locations on Previously Coated Surfaces
- ISO 8502 Preparation of steel substrates before application of paints and related products – Tests for the assessment of surface cleanliness
- Part 5: Measurement of chloride on steel surfaces prepared for painting–Ion detection tube method (ISO 8502-5:1998)
- Part 6: Extraction of soluble contaminants for analysis – The Bresle method (ISO 8502-6:1995)
- Part 9: Field method for conductometric determination of water-soluble salts (ISO 8502-9:1998)
- Part 10: Field method for the titrimetric determination of water-soluble chloride (ISO 8502-10:1999)
As a coating inspector, it is important to use the extraction and analysis methods specified for the project and not just the one you are comfortable with (or were trained on). It is also important to recognize that there is no industry-wide acceptance level. Tolerance levels can range from non-detectable to 25 or even 50 µg/cm2 (micrograms per square centimeter) and may vary by the type of contaminant (e.g., chloride versus sulfate). The project specification should indicate the maximum surface concentration for each type of anionic contamination. If ion-specific testing (i.e., salt-specific testing such as chloride or sulfate) is not required, then a maximum conductivity threshold may be specified, such as 5 µS/cm (micro-Siemen per centimeter). Conductivity measurements assess contamination from any and all soluble salts that are present, regardless of the specific type of salt(s) involved.
Testing Procedures
SSPC Guide 15 contains a table that overviews compatible methods for retrieval (extraction) and analysis of soluble salts from steel and other non-porous surfaces. It addresses measurements by conductivity (not ion-specific) and ion-specific methods. For conductivity measurements, there are two meters (Soluble Salt Meter and Surface Salinity Meter) that perform the extraction and analysis in a self-contained unit using integrated conductivity sensors. Each uses reagent water for the extraction process and the entire process is automated.
More commonly, though the procedures for extraction or retrieval of the salts from the surface and the method of analysis are conducted separately; the table in Guide 15 refers to these as multi-step conductivity and multi-step ion-specific methods. Many of these are listed below. Note that step-by-step instructions are not included in this article but are generally covered in Guide 15 and specifically addressed by the test kit manufacturers.
Methods of Extraction
The methods of extraction or retrieval include:
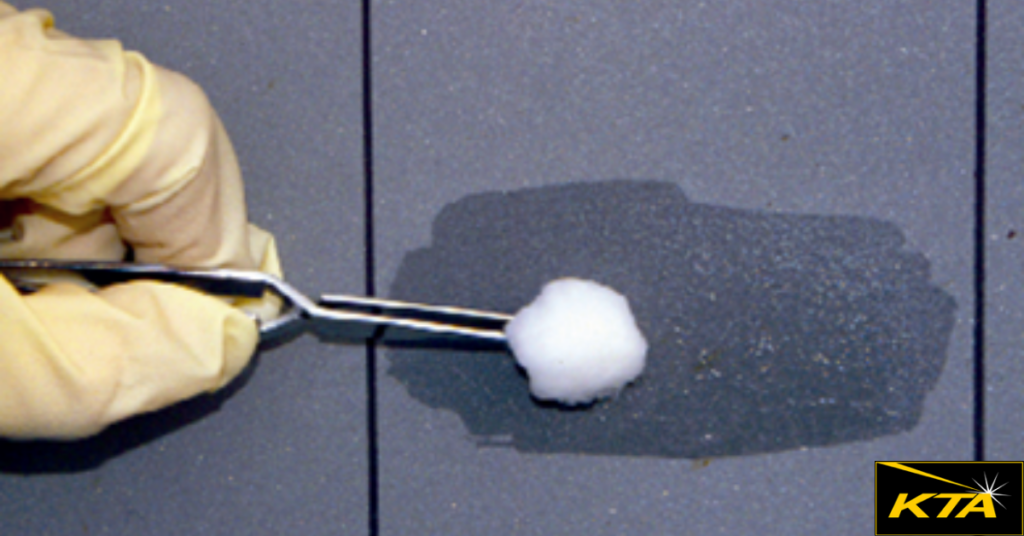
1. Surface swabbing (i.e., swabbing a known surface area [e.g., 100 cm2] with a saturated cotton ball using a premeasured quantity of reagent water [e.g., 10 mL])

2. Adhesively bonded latex or polyurethane patch or reusable flexible magnetic cell (i.e., attaching an adhesive latex/polyurethane patch or reusable, flexible magnetic cell with a known cell size [12.25 cm2] to the surface and injecting a premeasured quantity of reagent water [3 mL] into the latex patch using a syringe)



3. Adhesively bonded latex sleeve (i.e., pouring a premeasured quantity of proprietary extraction solution [10 mL] into a latex sleeve with a known opening size [10 cm2] and attaching the sleeve to the surface



4. Saturated filter paper (i.e., known size paper is saturated with reagent water and placed onto the surface)


Methods of Analysis
The methods of analyzing the extract, or test solution, are categorized by anion type. Surface concentrations are calculated using the formula:
(PPM x mL of extraction liquid) ÷ area extracted in cm2 = micrograms/cm2 (or µg/cm2)
PPM: Part per Million
mL: Milliliter (same as cubic centimeter, or cc)
µg: Microgram cm2: Square Centimeter
Note: When the amount of extraction liquid (in mL) is the same value as the area extracted (in cm2), there is no calculation necessary since these two values will cancel one another.
Example: (32 PPM x 10mL) ÷ 10 cm2 = 32 micrograms/cm2
Chloride Ion
1. Chloride ion indicator strips. The strips contain silver dichromate, which precipitates white in the presence of sodium chloride in the test solution. The numerical value on the test strip is converted to PPM using a conversion chart on the test strip container.



2. Chloride ion detection tubes. Kitagawa tubes contain silver dichromate, which precipitates white in the presence of sodium chloride in the test solution. The numerical value on the tube is PPM.


Ferrous Ion
1. Ferrous ion indicator strips. The test strip is dipped into the test solution, allowed to react for 10 seconds, then compared to a color indicator chart on the test strip container. The unit assigned to each color is milligrams/liter Fe2+, which is the same as PPM Fe2+.


Nitrate Ion
1. Nitrate ion indicator strips. The test strip is dipped into the test solution and compared to a color indicator chart on the test strip envelope. The unit assigned to each color is PPM NO3-1.

Sulfate Ion
1. Sulfate ion by Colorimetric method. The test solution is filtered into a special clear glass vial, capped, and inserted into a sulfate detection meter and “zeroed.” A premeasured quantity of Barium Chloride is added to the solution, the vial is recapped, shaken, and inserted into the meter. The value displayed on the meter is PPM SO4-2



Non-ion Specific (Conductivity)

1. Probe-type conductivity meters. The solution is dispensed onto the probe. The value displayed is in micro-Siemen/cm (µS/cm) but can also be displayed in PPM and % salinity. Some manufacturers program a conversion into the meter so that the value is displayed in µg/cm2 or mg/m2 (based on ISO 8502-9, which is explained in Appendix A of Guide 15).
2. Concentric Ring conductivity meters. The saturated filter paper is transferred from the surface onto the conductivity meter. The value displayed (salt density) is in micro-Siemen /cm (µS/cm) but can be displayed as µg/cm2, mg/m2, PPM, or % salinity.

3. Grid-Electrode Array conductivity meters. The saturated filter paper is transferred from the surface onto the conductivity meter. The value displayed (salt density) is in micro-Siemen /cm (µS/cm) but can be displayed as µg/cm2, mg/m2, PPM, or % salinity.



Frequency of Testing
Neither SSPC Guide 15 nor the ISO 8502-3 (parts 5, 6, 9, 10) address the frequency of soluble salt testing. The project specification may address the number of locations to sample, for example, “Three tests for the first 1000 square feet blast cleaned each shift plus one test for each additional 2000 square feet or part thereof.” When this is the case, the coating inspector tests at this frequency.
NACE SP-0716-2016, “Soluble Salt Testing Frequency and Locations on Previously Coated Surfaces” may be invoked by contract. When this is the case, the following testing frequency is required:
- Five tests in the first 1000 square feet of prepared steel
- Two tests in the second 1000 square feet of prepared steel
- One test in each additional 1000 square feet of prepared steel
In addition, sampling is suggested in locations where pitting/section loss, corrosion, and/or coating failure is evident. Residual salts will more likely be present in these locations compared with smooth, uncorroded areas. Note that contamination levels will likely vary considerably from location to location, so repeatability/reproducibility of measurements is not available in the Standard Practice.
SSPC Guide 24, “Soluble Salt Testing Frequency and Locations on New Steel Surfaces” may be invoked when new steel is required to be tested prior to surface preparation and painting. The location and number of test sites are based on the shape and complexity of the components. Guide 24 addresses I-shaped components (I-beams), channels and angles, tanks, and vessels, pipe, and assembled structures. The number of components to sample is based on the batch size of the components being prepared and coated at the same time. There is an initial batch quantity and, based on the salt concentrations detected subsequent batch quantities. For more detail on sampling of new steel components see Tables 1, 2 and 3 in SSPC Guide 24.
Summary
This article described the importance of surface soluble salt testing and explored the various methods of extraction and analysis that can be performed in the shop or field. It also discussed various standards addressing the frequency of testing for both new and existing steel structures. As trained/certified coating inspectors we are expected to know how to perform a variety of extraction (retrieval) and analysis procedures, both ion-specific and via conductivity when surface soluble salt thresholds are invoked by specification. We are also expected to access, read, and comprehend industry standards published by legacy NACE and SSPC, as well as new AMPP standards when invoked by specification so that testing and reporting are conducted properly and at the frequency required. If you have questions about soluble salt testing procedures, KTA’s subject matter experts are only a telephone call or click away.
[1] Cleanliness of Recycled Ferrous Metallic Abrasives




Very informative. Easily comprehendible. Good read! Thank you.
We’re glad you found it helpful – thanks for the feedback!
thank you so much for share this kind of information, is very useful in the industry
We’re glad you found it helpful!