Powder coating metallic surfaces for corrosion protection and aesthetic purposes has grown by leaps and bound in the United States and more so in other parts of the world. The use of powder coating on aluminum and steel sheet products has led the way to greater technology and reduced costs as the demand has grown. Powder coating has long had traction for galvanized items but often the learning process resulted in a step back as steps forward were made. A major reason was that failures ocurred and it was not always evident how to avoid them in the surface preparation and application processes. This article provides some insight to the powder coating process for galvanizing and begins with a brief discussion of what powder coatings are and are not, as well as some of the characteristics of hot dipped galvanized steel that makes it difficult to properly coat.
Powder Coating
Powder coatings can be divided into two categories, thermoplastic and thermoset, which are essentially the same as non-convertible and convertible liquid coatings, respectively. Thermoplastic powders maintain their chemical identity since it does not change when the dry film forms. Rather than curing by solvent evaporation, the film solidifies upon cooling. Thermoset products do undergo a chemical change (are converted) as they cure and solidify. Simply stated, thermoplastic powder melts into a liquid when heated and cools into a film of the same chemical form. Thermoset powder coatings are not [fully] cured but upon heating melt, the heat provides energy needed for chemical crosslinking to occur and cures (and cools) into a new polymer.
Thermoplastic Powders (convertible)
Nylon, Polyester, Polyolefins, Polyvinyl Chloride, Polyvinylidene Fluoride
Thermosetting Powders (non-convertible)
Acrylic, Epoxy, Epoxy-Polyester Hybrid, Polyester TGIC, Urethane Polyester
The powder is the result of a melt-mix of ingredients that include the resin system and many of the functional additives employed for liquid coating that influence flow, leveling, color and cure. The mixed material is ground to a fine, flour-like powder and packaged.
The commercial use of powder coating developed initially in Europe, where thermoplastic materials in powder form where being flame sprayed on to metallic surfaces. The fluidized bed process developed by Dr. Gemmer in Germany was patented in 1953 and remained the mainstay of the application process into the early 1960s when electrostatic spray became available. The most common thermoplastic materials of the 1940s and early 1950s were cellulose acetate butyrate (CAB), nylon 11 (a grade developed in France), polyester, plasticized polyvinyl chloride (PVC) and a few others. Thermoset epoxy also became available during this time.
Application
Powder coatings, as the name states, are powders not liquids. However, for them to become effective as protective and attractive finishes they must form a continuous film on the items being coated. They necessarily go through a liquification process by use of heat energy. Powders can be flame sprayed, applied using fluidized beds and sprayed electrostatically.
- Flame spray application is accomplished by using powder from a feed hopper into a (clean) air stream to carry powder through a fueled flame[1] that melts (liquifies) the atomized liquid droplets and are applied to the piece being coated. The coating flows out creating a continuous film onto pieces that are often pre-heated to allow for continued flow. The film solidifies as it cools below the melting (gel) point. The temperature of the item, duration of application and cooling rate serve to determine the film thickness of the coating. Thicknesses of 8 to 10 mils are normally required to achieve a pinhole free film. Generally, thicknesses are limited to about 25 mils. Application rates of 50 square feet (SF) per hour to achieve 10-15 mils of coating are common. Flame spray is used in the field as the primary means of application or to effect repairs in the field for shop powder coated items.
- Fluidized bed application also involves pre-heating the item to be coated, then lowering the item into a cloud of powder suspended by air jets. The heat content of the item and time in the fluidized powder established the thickness of the film formed on the surface. The thickness builds rapidly at first but generally approaches a maximum within 10 seconds. Typical thicknesses are 10-20 mils although multiple heating and dipping cycles can produce thicknesses of 100 mils. High mass [steel] items typically contain enough heat to produce a fused coating film. Low mass items (screens, tubes) may require post heating to complete the fusion and cure. Electrostatic fluidized beds are also available, generally for small and two-dimensional parts (wire screens). The most commonly used powder is epoxy although some thermoplastic resins may be applied in this manner. The powder cloud is charged using ionized air and the particles deposited on the grounded piece. This technique is widely used to insulate electric motor rotors[2] and allows the removal of powder by vacuum from surfaces that are not to be powder coated.
Electrostatic spray of powder coating, like electrostatic spray of liquid coatings, involves air spray of electrically charged [dry] particles onto grounded pieces. A feed hose carries the powder (or liquid) to the spray gun which discharges the particles through a corona discharge imparting a charge to the powder particles which are propelled to the grounded object where they are deposited. The electric attraction is based on the electric potential and influences the thickness of the powder layer on the surface. The coated pieces may be pre-heated (such as FBE on pipe) but typically go through a post application heating to liquify the coating and then go through a cooling process. The process is easily automated for production facilities that can make use of several designs of robotic application nozzles.
Alternatively, the application may be performed by individuals using electrostatic spray guns for piece work.
The keys to proper film formation by powder coating are coverage, temperature and time. Manufacturers provide instructions for proper heating temperatures; temperature hold times and cooling steps that may be necessary. Misapplications can occur due to temperature being too low or too high, improper heating periods and uneven coverage of the powder. As with liquid coatings applied electrostatically, Faraday cage effect” can result in low coverage in corners and edges.
Hot Dipped Galvanizing
The American Galvanizers Association[3] is an excellent source for information about the galvanizing processes, standards, inspections, testing and what constitutes a properly galvanized product. Note however, properly galvanized does not mean paintable. A short discussion on what the galvanizing process is, for those not already familiar with galvanizing, may be useful.
Galvanizing is the process used to apply a metallurgically bonded layer of zinc [metal] to properly cleaned steel plates, rods, shapes and articles. The zinc [galvanizing] includes several metallurgically distinct layers and function as a protective coating layer by being both corrosion resistant and anodic (sacrificial) to steel in wet environments. The layers that form begin with a gamma layer about 21-28% iron (balance zinc) just above the base steel and additional layers (delta and zeta) having lesser amounts of iron until an essentially pure zinc layer (eta) forms on the top. See Figure 1.
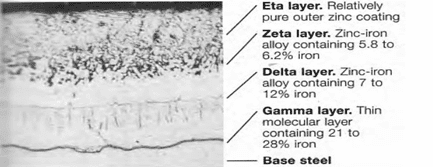
Steel surfaces are typically prepared and galvanized in the sequence shown in Figure 2. Grease, oils and soils are removed in alkali solutions after which the steel is rinsed and subject to pickling to remove rust, mill scale, and other foreign matter. The steel is rinsed again, immersed in a flux bath removed and allowed to dry. In alternative processes, the flux solution floats on the top of the molten zinc and the steel item receives the flux layer as it enters the hot dip tank to be galvanized. An alternative to pickling is abrasive blast cleaning to a degree necessary for coating, as described in SSPC-SP 16[4].
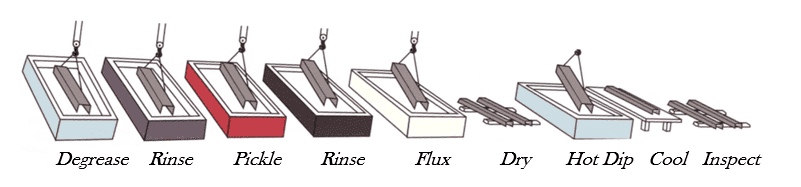
A “wet” galvanizing process follows the same steps except the flux lies on the
zinc bath and the piece passes through the flux into the molten zinc.
Following galvanizing the items may be quenched with water or water solutions containing rust inhibitive surface treatments or may be air cooled without surface treatment. Inspection of the galvanized items should be performed to ensure conformance with the specified galvanizing standard. However, it important to recognized that a properly galvanized surface is not the same as a galvanized surface properly prepared for painting or coating. The requirements are different and critical to successfully produce a painted galvanized product.
Powder coat can be applied to a variety of surfaces, including ferrous and non-ferrous metals, plastics, fiberboard and wood. Ferrous metal is by far the most common; consider all the fusion bonded epoxy pipe installed each year. Hot dip galvanizing has become quite common for light posts, traffic signals and sign trusses, handrails, etc. in cities and along highways. These are commonly powder coated.
Painting Hot Dipped Galvanizing
The “go-to” document for painting hot dip galvanizing in the United States has historically been ASTM[5] D6386, “Standard Practice for Preparation of Zinc (Hot-Dip Galvanized) Coated Iron and Steel Product and Hardware Surfaces for Painting”. A 2010 standard, SSPC[6]-SP 16, “Brush-Off Blast Cleaning of Coated and Uncoated Galvanized Steel, Stainless Steels, and Non-Ferrous Metals” references ASTM D6386. These documents address application of liquid coatings. ASTM D7803, “Standard Practice for Preparation of Zinc (Hot-Dip Galvanized) Coated Iron and Steel Product and Hardware Surfaces for Powder Coating” addresses powder coating. Even though this standard is specific to powder coating of galvanized steel it contains the same cleaning and preparation techniques for the surfaces coated with liquid products as ASTM D6386 and SSPC-SP 16. However, ASTM D7803 includes a pre-heating step to drive off air and moisture from the prepared galvanized surface prior to powder coating. This step is described as appropriate and necessary to prevent pinholes and blisters in the film. Solvent entrapment is not an issue since the coating products are solvent free. However, recall that the powder coating process requires that the powder particles must be converted into a liquid so that cross-linking (thermoset resins), flow-out and wetting (thermoset and thermoplastic resins) occur to achieve coverage (continuity) and adhesion. The pre-bake step is recommended to be about 70OF above the curing oven temperature.
Following the pre-bake step allowing the surface temperature to come down to room temperature before coating and curing could be self-defeating. However, the items should be cooled to below the melt/cure temperatures of the powder coating before application. The pre-bake step will also mollify non-visible salts (which are hygroscopic) and volatilize associated surface bound water molecules.
Review of the above references helps illustrate that hot dip galvanizing has always been difficult to properly paint. This is directly related to the surface chemistry of galvanizing. Metallic zinc is a reactive surface[7] which is easily oxidized upon exposure to air and moisture. Zinc oxides and hydroxides that form on the surface interfere with adhesion and do not inhibit further surface oxidation. Wet environments promote the formation of zinc corrosion products (e.g. white storage stain). A period of up to two years in atmospheric service is necessary for the gray zinc patina (zinc carbonate; ZnCO3) to form over the zinc surface. This zinc salt is insoluble in water and inhibits further corrosion (oxidation) at the zinc metal surface below. It is well bonded to the surface and is considered a good substrate film for painting. Coating recommendations are that new galvanizing be coated within 48 hours following initial galvanizing. Following that period and until the patina is fully formed the zinc surface requires thorough cleaning and/or roughening to remove zinc oxides and hydroxide.
ASTM D6386 − 16a Section 6.1 states “In some atmospheric conditions, such as high humidity or high temperature, or both, the formation of zinc oxide on the blasted surface will begin very quickly so the paint coating should be applied within 30 min after sweep blasting. Zinc oxide formation is not visible to the naked eye; therefore, in any atmosphere, painting should be as soon as possible after surface preparation.”
This same language is found in ASTM D7803, “Whenever galvanized steel is rinsed, it is desirable to use heated drying to accelerate the complete removal of water from the surface.” and “ Powder coating shall take place soon after treatment to avoid pick up of surface contaminants.”
The galvanizer should be notified the finished pieces are going to be painted and what handling and treatment should be avoided. Both ASTM standards caution about water quenching and/or chromate conversion coatings following galvanizing. These treatments render the surface unsuitable for coating adhesion.
SSPC-SP 16 in the non-mandatory Appendix, Section A9.2 Zinc Oxides: states, “Newly exposed zinc surfaces will oxidize rapidly, especially in the presence of moisture. During brush-off blast cleaning and subsequent painting of galvanized steel, the surface temperature should be a minimum of 3 °C (5 °F) above the dew point, in order to retard the formation of zinc oxides. To limit the amount of zinc oxide on the cleaned surface, galvanizing should not be permitted to get damp after cleaning and should be painted as soon as possible within the same work shift that the surfaces were cleaned.”
The urgency for coating galvanized steel so soon after surface preparation is a testament to how reactive zinc metal is.
Clearly, there are a multitude of steps to produce a properly powder coated galvanized item. This should not be lost on specifiers or purchasers.
From a design standpoint, the key to producing a properly powder coated galvanized product is to remember: A properly galvanized surface is not the same as a galvanized surface properly prepared for painting. There are too many galvanizers and too many powder coaters that do not understand this.
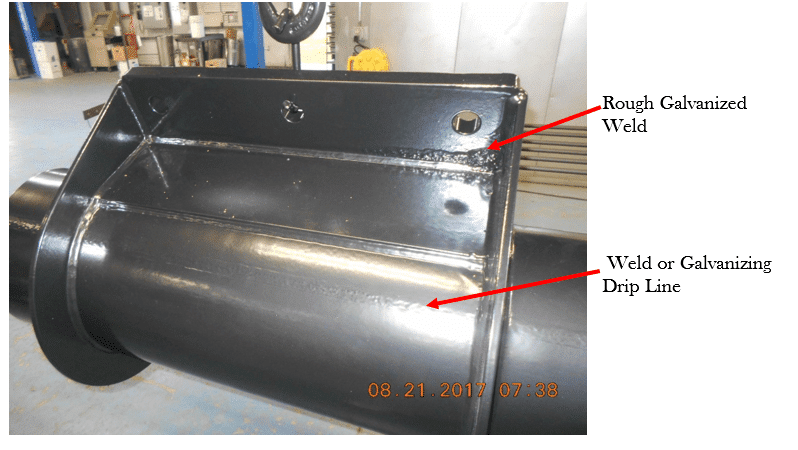
Case in point: The images below show powder coated surfaces. There is a weld that was very rough but galvanized without dressing it. Is there a continuous [powder] coat film? Visually, what other surfaces raise concerns?
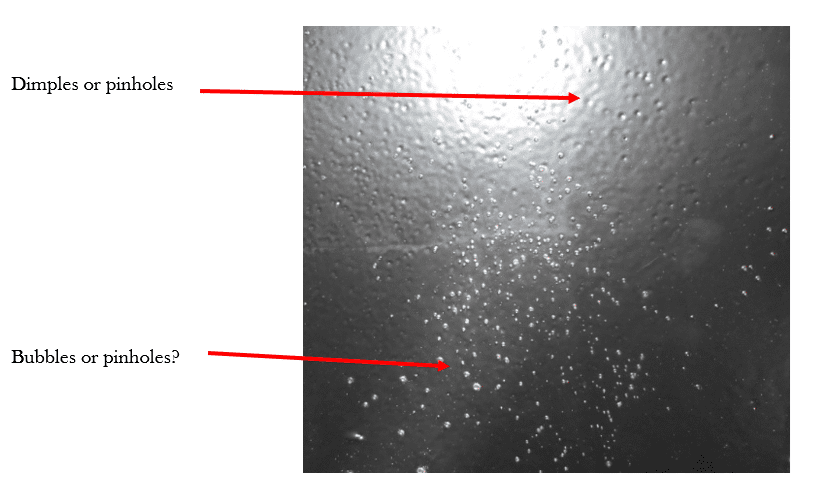
Was the item below properly pre-baked? Coated? Cured? Is the appearance simply an aesthetic issue or is there also a risk of reduced corrosion protection?
When the opportunity arises to review a powder coating specification make note of how infrequently holiday testing is required and when multiple coats are required.
Summary
Painting galvanizing has long been a difficult task with liquid coatings applied to inadequate surface preparation or aging until the carbonate patina has formed. Careful surface cleaning and removal of zinc oxides and hydroxides on the surface,- due to the reactivity of metallic zinc, when not properly done doom the applied organic coatings to failure. Powder coating required the same degree of cleaning and preparation as liquid coating plus the additional step of preheating to remove vapor and gases that would be trapped by the powder melt process leading to pinholes, bubbles and eventual delamination. There are numerous advantages to powder coating (VOC, wasted materials, etc.) the challenges to successfully coating galvanized steel with organic films are no less complex.

About the Author:
Rich Burgess is a senior consultant for KTA-Tator, Inc. where he has been employed for over 23 years. He is a member of SSPC and NACE and an active committee member for joint standards. Burgess is an SSPC-Certified Protective Coatings Specialist, a NACE-Certified Coating Inspector Level 3 (Peer Review) and an SSPC C-3 Supervisor/Competent Person for Deleading of Industrial Structures. In his current position, he performs coatings evaluations, coating failure analysis, specification preparation, expert witness and project management services for clients in the transportation, power generation, water/wastewater, shipping, marine and aerospace industries. Burgess is a principal instructor for the SSPC C-1, C-3, and C-5 courses, for the NACE CIP Program and a variety of KTA-offered training seminars. He holds a Bachelor of Science degree in Environmental Science from Rutgers University and a Master of Science in Operations Management from the University of Arkansas.
[1] Propane and oxygen/acetylene are commonly used.
[2] Powder Coating Institute (PCI), Powder Coating: The Complete Finisher’s Handbook, 3rd Edition. PCI Alexandria, VA. (www.powdercoating.org)
[3] American Galvanizers Association (AGA), Centennial, CO 80112, https://www.galvanizeit.org/
[4] Brush-Off Blast Cleaning of Coated and Uncoated Galvanized Steel, Stainless Steels, and Non-Ferrous Metals
[5] ASTM International, West Conshohocken, PA 19428-2959, http://www.astm.org
[6] Society for Protective Coatings (SSPC) Pittsburgh, PA 15222-4656, http://www.sspc.org.
[7] Metals in the electromotive series, the most easily oxidized (reactive) to least easily oxidized is lithium, potassium, calcium, sodium, magnesium, aluminum, zinc, chromium, iron, cobalt, nickel, lead, hydrogen, copper, mercury, silver, platinum, gold.



I’m glad you talked about how they need to develop a continuous film on the items being coated in order to function as protective and appealing finishes. Before the new year, I’ve been considering repainting my work trailer. Due to its low cost and environmental friendliness, powder coating seems like a good choice for this project.